Epigraph Vol. 23 Issue 1, Winter 2021
Joint task force reassures clinicians, patients after US lamotrigine warning
By Nancy Volkers, ILAE communications officer
Following a recent safety warning issued by the US Food and Drug Administration (FDA) on lamotrigine, an ad hoc joint task force of the ILAE and American Epilepsy Society (AES) published an advisory for health care professionals worldwide that discusses how to minimize cardiac-related safety risks in patients taking lamotrigine.
Task force members include neurologists with expertise in epilepsy, pharmacology, and clinical neurophysiology; a cardiologist with expertise in investigating the cardiac effects of anti-seizure medications; and an expert on mechanisms affecting neuro-cardiac electrical function.The advisory is not meant to replace regulatory requirements, but to provide information and reassurance to clinicians and patients.
Guidance and reassurance
In October 2020, the FDA added two paragraphs to lamotrigine’s drug labeling (see box). The paragraphs state that lamotrigine may increase risk for arrhythmia in people with heart conditions. The warning is based on unpublished in vitro (laboratory) data from drug manufacturer Glaxo Smith Kline, which produces the brand name version of lamotrigine (Lamictal).
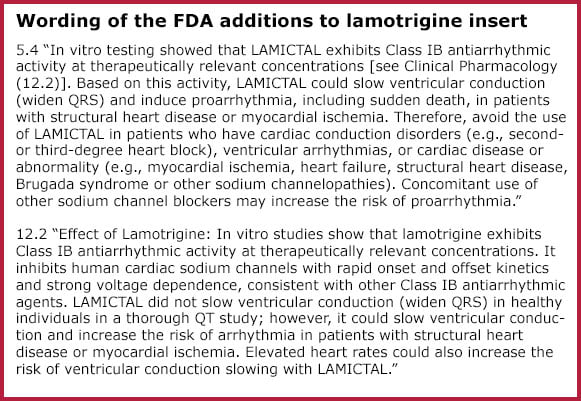
The FDA guidance says the use of lamotrigine should be avoided in “people who have cardiac conduction disorders, ventricular arrhythmias, or cardiac disease or abnormality.”
“The warning essentially says to avoid lamotrigine in anyone with any kind of cardiac disorder – this is very strong terminology. However, all the evidence we have from clinical studies does not point to any special cardiac risk,” said Emilio Perucca, a neurologist and clinical pharmacologist at the University of Pavia, Italy, and co-chair of the ILAE/AES task force. (See references to clinical studies below.)
He noted that drug labels for other sodium channel blockers, including carbamazepine and lacosamide, have wording that mentions concern for people with certain cardiac conditions. “But there is no antiseizure medication other than lamotrigine, and no agency other than the FDA, that has such a strong warning recommending avoidance across the board in people with any cardiac disease,” he said.
Task force recommendations

The label change has caused some surprise, said Perucca, partly because other regulatory entities have not followed suit. “We know that the European Medicines Agency [the European equivalent of the FDA] is aware of the issue but so far they have not taken any action to recommend a label change.”
The ILAE/AES task force does not see a strong basis to recommend avoiding the use of lamotrigine in any person with a cardiac condition, said Perucca. “There is no major reason to change clinical practice, other than being more cautious in people who have certain cardiac disorders. In particular, physicians may consider doing an EKG before prescribing lamotrigine in patients known to have, or at special risk for, certain cardiac diseases.”
The highest-risk groups are people with second- or third-degree heart block, Brugada syndrome, arrhythmogenic ventricular cardiomyopathy (ARVC), left bundle branch block (LBBB), and right bundle branch block (RBBB) with left anterior or posterior fascicular block. For these groups, said Perucca, it would be wise to use an alternative antiseizure medication devoid of potential cardiac effects, if one is available. If no suitable alternative medication exists, a thorough cardiological investigation would be needed to determine if lamotrigine can be given safely. Consideration also should be given to potential interactions with other sodium channel blockers.
Background on lamotrigine
Lamotrigine is FDA approved to treat epilepsy and bipolar disorder. It is considered by many neurologists to be a treatment of choice for epilepsy with onset in the old age, said Perucca, because it is well tolerated and has a lower risk of affecting the blood levels of other medications commonly taken by older people.
“Lamotrigine is often given to older people, who often have concomitant cardiac conditions,” he said. “We want to reassure people who are on lamotrigine that there is no reason to discontinue it.
If you are concerned [about the FDA warning], talk with your doctor. In particular, you should seek medical advice if you have been experiencing feelings of fainting, or episodes of actual fainting. ”
Read the full advisory by the ILAE/AES Joint Task Force, "FDA Safety Warning on the Cardiac Effects of Lamotrigine"
References for clinical studies that found no elevated cardiac risks for lamotrigine:
Saetre E, Abdelnoor M, Amlie JP, Tossebro M, Perucca E, Taubøll E, Anfinsen OG, Isojärvi J, Gjerstad L. Cardiac function and antiepileptic drug treatment in the elderly: a comparison between lamotrigine and sustained-release carbamazepine. Epilepsia. 2009 Aug;50(8):1841- 9.
Dixon R, Job S, Oliver R, Tompson D, Wright JG, Maltby K, Lorch U, Taubel J.
Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects. Br J Clin Pharmacol. 2008 Sep;66(3):396-404.
Dixon R, Alexander S, Brickel N. Effect of lamotrigine on the PR interval in healthy subjects. Br J Clin Pharmacol. 2011 Jun;71(6):961-2.
Sveinsson O, Andersson T, Mattsson P, Carlsson S, Tomson T. Pharmacologic treatment and SUDEP risk: A nationwide, population-based, case-control study. Neurology. 2020 Nov 3;95(18):e2509-e2518
Subscribe to the ILAE Newsletter
To subscribe, please click on the button below.
Please send me information about ILAE activities and other
information of interest to the epilepsy community